By Hannah McGrath, MLML Biological Oceanography Lab
Plastic pollution continues to be a growing issue on our planet, especially for our oceans. The global pandemic only contributed to our growing plastic problem. During the height of the pandemic, I remember walking along Riverside Park in New York City to escape my tiny apartment; the sidewalks and shorelines were littered with KN95 masks and light blue latex gloves. As I continued my walks throughout the pandemic, the sight of personal protective equipment scattered across the city became the norm. According to lead researcher Dr. Patrício Silva at the University of Aveiro, the pandemic dramatically increased the amount of plastic medical waste that has entered our aquatic systems. These plastics can then degrade into microplastics (< 5 mm in size) through physical, chemical, and biological processes which can have adverse effects on ecological and human health.
Although microplastics are small in size, they have a disproportionate effect on the environment. For instance, zooplankton which are important players in our ocean food webs and the biological carbon pump, a process that exports carbon to the deep sea, are threatened by microplastics. Zooplankton are able to consume microplastics which can damage their intestinal tracts, alter gene expression, delay growth, and impact feeding behavior resulting in decreased reproductive abilities according to lead scientist Dr. Meiting He at the College of Marine Sciences, South China Agricultural University. Unsurprisingly, microplastics have been identified in the gut content of organisms’ at almost all trophic levels from zooplankton to humans. Microplastics are in the clothing we wear, seafood we consume, beauty products we use, and more. In fact, in a 2019 study lead author Kieran Cox, a PhD candidate at the University of Victoria, estimated that ~39,000-52,000 pieces of microplastic are ingested by humans annually!
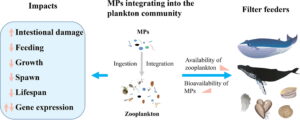
Illustration of microplastics (MPs) entering aquatic systems and being consumed by zooplankton resulting in the trophic-transfer of MPs up the food chain (He et al 2022).
Not only is plastic pollution increasing but so is our need to adopt effective and sustainable ways for disposing plastics at a large scale. Current methods for plastic disposal are mismanaged and unsustainable. One common way to dispose of plastic is by incineration. However, during incineration plastics release carcinogens, dioxins, furans, heavy metals and sulfides into the environment states researchers Dr. Aubrey Chigwada and Dr. Memory Tekere at the University of South Africa. Another common method is dumping plastic waste into landfills but this causes plastic overflow affecting the biodiversity of the region. In addition, landfills store not only plastic waste but all types of waste that can decompose. During decomposition processes the potent greenhouse gas, methane, is released into the atmosphere which contributes to climate change. These landfills can also leak which can contaminate nearby groundwaters. Although recycling may seem like a promising way to dispose of plastics, at large scales it is too expensive and not feasible.
A more sustainable method to dispose of plastic is using microorganisms like bacteria that can biodegrade plastics. The first study that investigated microplastic degradation by microorganisms was Dr. Cacciari and his colleagues from the University of Tuscia in 1993. The researchers used the bacteria Pseudomonas and Vibrio to degrade polypropylene. Since 1993, many researchers have studied biodegradation of various plastics using bacteria from around the globe. Bacteria naturally exist in various environments from cow dung to human eyelashes to hot springs to polar ice caps making them suitable candidates for degrading microplastics. For instance, lead author Jun Yang at Beihang University, Beijing found two bacterial strains isolated from the gut of Indian mealmoths that were able to consume the plastic polyethylene.
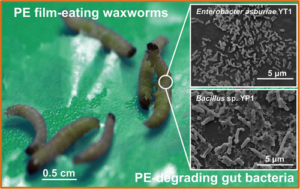
Image of the two bacterial strains, Enterobacter asburiae and Bacillus sp. isolated from the gut of Indian meal moths (Yang et al. 2014).
Not only can bacteria naturally degrade plastics, but they can also be geoengineered to remove plastic from our oceans. Bacteria may just be nature's tiny heroes to combat plastic pollution. Currently, Professor Song Lin Chua and his colleagues at the Hong Kong Polytechnic University (PolyU) have bioengineered the bacteria Pseudomonas aeruginosa to remove microplastics from the environment. The researchers plan to use the sticky nature of bacteria to create “tape-like microbe nets” to capture microplastics. These microbial nets filled with microplastics then sink to the bottom of the water column. The bacteria’s biofilm dispersal gene is then engineered to release these microplastics from the biofilm traps. The bulk microplastics then float to the surface and are recycled. These preliminary experiments have been successful but have not been conducted outside of a controlled setting.
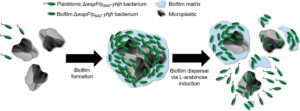
Schematic illustration of the bioengineered bacteria, Pseudomonas aeruginosa, removing microplastics from the water column using the 'capture-and-release' method developed by researchers at Hong Kong Polytechnic University
Although scientists are developing innovative ways to remove plastics from our ocean, there have been concerns about using bacteria to do this. Engineering bacteria to break down plastics especially in hot spots like the Pacific Garbage patch may reduce plastic waste, but may also have unintended consequences. For instance, breaking down microplastics may increase microplastic ingestion by other marine organisms like zooplankton that are known to consume microplastics. Another drawback is that the bacteria aeruginosa, that was used in PolyU preliminary experiments, carries diseases for humans’ states Professor Chua. Researchers are still searching for a bacterium that could be engineered that is natural and safe to humans at a large scale. But I am hopeful that scientists will find a safe and suitable candidate since bacteria are extremely abundant in the ocean. For every 1 ml of seawater there are ~1 million bacteria!
The reality is plastic pollution in the ocean is rapidly increasing. It is imperative that we find a solution to our growing plastic pollution problem sooner than later. Bacteria may just be one solution to our global plastic problem. However, more research and experimentation are still needed to understand the true benefits and consequences of genetically engineering bacteria to remove plastic from our oceans. Will bacteria be able to solve our plastic pollution problem?
References
Cacciari, I., Quatrini, P., Zirletta, G., Mincione, E., Vinciguerra, V., Lupattelli, P., Giovannozzi Sermanni, G., 1993. Isotactic polypropylene biodegradation by a microbial community: physicochemical characterization of metabolites produced. Appl. Environ. Microbiol. 59, 3695–3700. https://doi.org/10.1128/aem.59.11.3695-3700.1993
Chigwada, A.D., Tekere, M., 2023. The plastic and microplastic waste menace and bacterial biodegradation for sustainable environmental clean-up a review. Environ. Res. 231, 116110. https://doi.org/10.1016/j.envres.2023.116110
Cox, K.D., Covernton, G.A., Davies, H.L., Dower, J.F., Juanes, F., Dudas, S.E., 2020. Correction to human consumption of microplastics. Environ. Sci. Technol. 54, 10974–10974. https://doi.org/10.1021/acs.est.0c04032
He, M., Yan, M., Chen, X., Wang, X., Gong, H., Wang, W., Wang, J., 2022. Bioavailability and toxicity of microplastics to zooplankton. Gondwana Res. 108, 120–126. https://doi.org/10.1016/j.gr.2021.07.021
Liu, S.Y., Leung, M.M.-L., Fang, J.K.-H., Chua, S.L., 2021. Engineering a microbial ‘trap and release’ mechanism for microplastics removal. Chem. Eng. J. 404, 127079. https://doi.org/10.1016/j.cej.2020.127079
Patrício Silva, A.L., Prata, J.C., Walker, T.R., Duarte, A.C., Ouyang, W., Barcelò, D., Rocha-Santos, T., 2021. Increased plastic pollution due to COVID-19 pandemic: Challenges and recommendations. Chem. Eng. J. 405, 126683. https://doi.org/10.1016/j.cej.2020.126683
Yang, J., Yang, Y., Wu, W.-M., Zhao, J., Jiang, L., 2014. Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environ. Sci. Technol. 48, 13776–13784. https://doi.org/10.1021/es504038a